Description
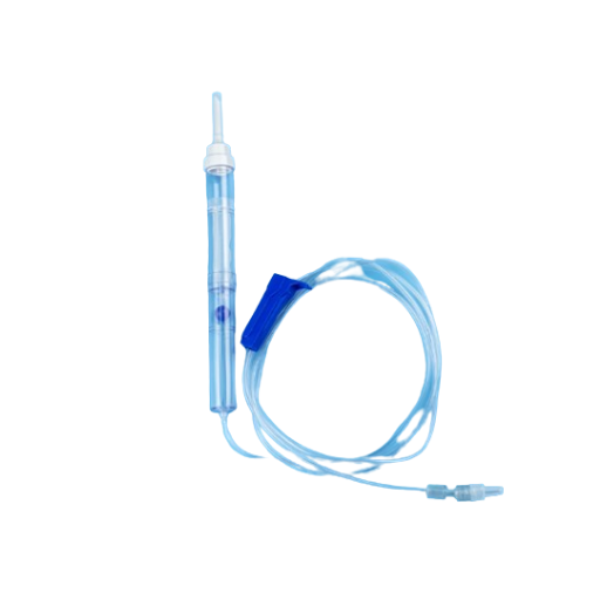
The transfusion sets are assembled by a closure-piercing device, a filter enclosed in a transparent chamber, a transparent drip chamber, a length of tubing with a flow regulator, a site for the injection of drugs, and a Luer connecter and a venous device. The transfusion sets are used for blood transfusion.
The blood transfusion sets prevent blood clots from entering into a patient. The blood transfer set provides precise results, ensures reliable blood transfusions.
Item No : 90183900
Order(MOQ) : 50000PCS
Payment : T/T,DP
Color : custom
Shipping Port : Shanghai/Nanjing/Shenzhen/Ningbo
Lead Time : 35days
Main Material: Medical grade PP,Medical grade PVC, Medical grade ABS
SIZE:TKX-00、TKX-01、TKX-02、TK-BT-01、TK-BT-02、TK-BT-03、TK-BT-04、TK-BT-07、TK-BT-08、TK-BT-09、TK-BT-10、TKBT-F01、TKBT-F02.
Intended Use
It is suitable for inputting the blood into the human veins under the action of gravity.
Characteristic:
20drops=1±0.1ml
Flexible drip chamber
Roller clamp, one/two spikes
Y-injection port
Slip or lock adapter
Air-inlet device
For gravity use
Sterile
Structure Properties Products
Safe blood transfusion design: The filtration rate of bloodclot is greater than 80%.Reliable regulator design: adjustable stroke greater than 30mm, can adjust the flow from zero to maximum, theadjust groove wall surface is thick, not easy to deform.and the adjustment is precise.
Multi-category design: PVC materials,and PVC DEHP FREE materials which are successively with better safety. It could meet the different needs of customers.
MODEL or TYPE Carton dimension/Q’TY(pcs) 40HQ Q’TY(CTN) 40GQ Q’TY(CTN) 20GQ Q’TY(CTN)
TKX-00、TKX-01、TKX-02、TK-BT-01、TK-BT-02、TK-BT-03、TK-BT-04、TK-BT-07、TK-BT-08、TK-BT-09、TK-BT-10、TKBT-F01、TKBT-F02. 58.5x46x45cm/400pcs 556 490 230
Notes
Product Conformance
Conform to ISO 1135-4 etc.;
In compliance with European Medical Device Driective MDD 93/42/EEC:1993( 2007/47/EC);
Quality Assurance
Manufacturing process is in compliance with ISO 13485 and ISO 9001 Quality System.

Reviews
There are no reviews yet.